How Are Mutations Repaired Bio
Learning Objectives
- Compare point mutations and frameshift mutations
- Describe the differences between missense, nonsense, and silent mutations
- Describe the differences betwixt lite and nighttime repair
- Explicate how different mutagens deed
- Explicate why the Ames exam tin exist used to notice carcinogens
- Clarify sequences of DNA and place examples of types of mutations
A mutation is a heritable change in the DNA sequence of an organism. The resulting organism, called a mutant, may have a recognizable change in phenotype compared to the wild type, which is the phenotype near commonly observed in nature. A change in the DNA sequence is conferred to mRNA through transcription, and may pb to an altered amino acrid sequence in a protein on translation. Because proteins carry out the vast majority of cellular functions, a change in amino acrid sequence in a protein may lead to an contradistinct phenotype for the cell and organism.
Furnishings of Mutations on Dna Sequence
There are several types of mutations that are classified according to how the DNA molecule is altered. Ane blazon, called a bespeak mutation, affects a unmarried base and most commonly occurs when ane base is substituted or replaced by some other. Mutations also issue from the addition of one or more bases, known every bit an insertion, or the removal of one or more bases, known as a deletion.
Think about It
- What type of a mutation occurs when a gene has two fewer nucleotides in its sequence?
Effects of Mutations on Protein Structure and Part
Point mutations may take a wide range of effects on protein function (Figure 1). As a consequence of the degeneracy of the genetic lawmaking, a signal mutation will commonly result in the same amino acid being incorporated into the resulting polypeptide despite the sequence change. This change would take no result on the protein's structure, and is thus called a silent mutation. A missense mutation results in a dissimilar amino acid beingness incorporated into the resulting polypeptide. The effect of a missense mutation depends on how chemically different the new amino acid is from the wild-type amino acid. The location of the changed amino acid within the protein also is important. For example, if the changed amino acrid is part of the enzyme's active site, and so the consequence of the missense mutation may be significant. Many missense mutations consequence in proteins that are still functional, at least to some caste. Sometimes the effects of missense mutations may be only apparent under sure environmental conditions; such missense mutations are called provisional mutations. Rarely, a missense mutation may be beneficial. Under the right environmental conditions, this blazon of mutation may give the organism that harbors it a selective advantage. Yet another type of point mutation, called a nonsense mutation, converts a codon encoding an amino acid (a sense codon) into a cease codon (a nonsense codon). Nonsense mutations upshot in the synthesis of proteins that are shorter than the wild type and typically non functional.
Deletions and insertions also crusade various effects. Considering codons are triplets of nucleotides, insertions or deletions in groups of three nucleotides may lead to the insertion or deletion of one or more than amino acids and may not cause pregnant furnishings on the resulting protein'due south functionality. However, frameshift mutations, caused by insertions or deletions of a number of nucleotides that are not a multiple of three are extremely problematic because a shift in the reading frame results (Figure i). Because ribosomes read the mRNA in triplet codons, frameshift mutations tin can change every amino acid subsequently the point of the mutation. The new reading frame may likewise include a stop codon before the terminate of the coding sequence. Consequently, proteins made from genes containing frameshift mutations are about always nonfunctional.
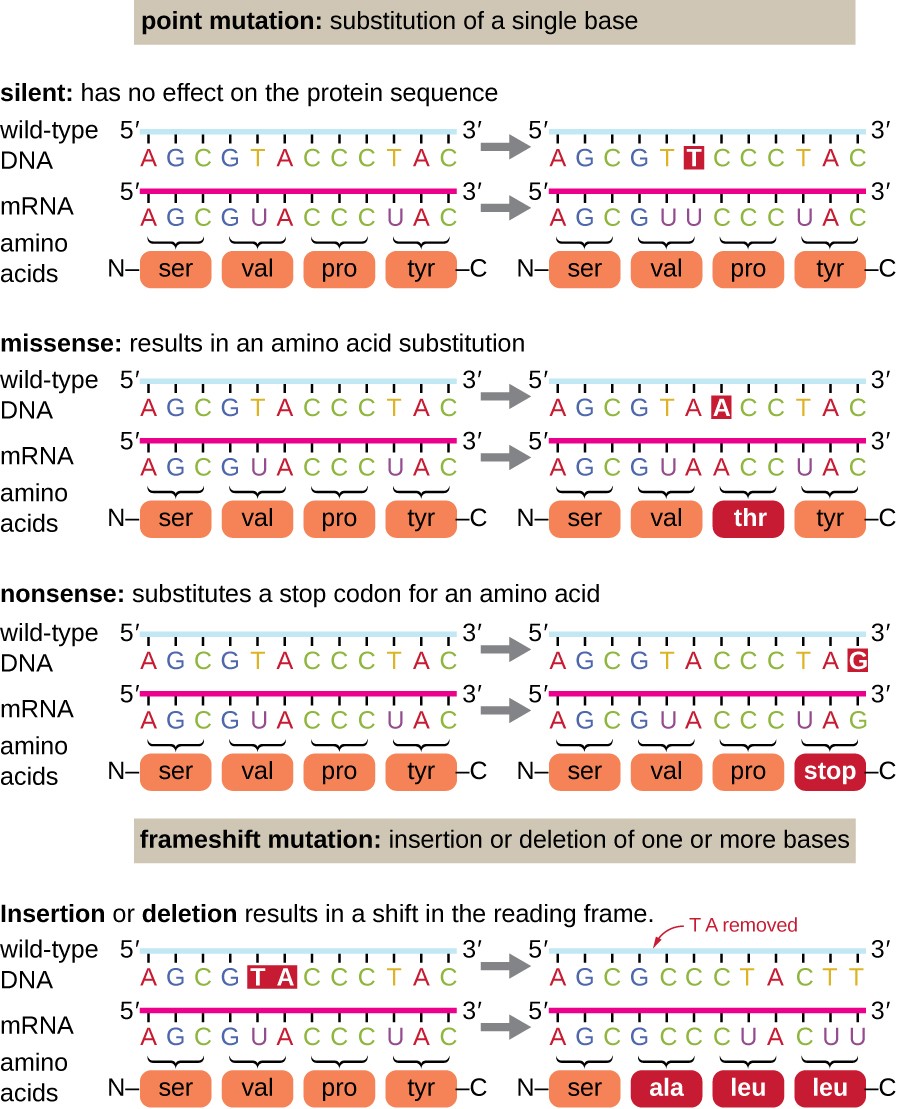
Effigy 1. Click for a larger paradigm. Mutations can atomic number 82 to changes in the protein sequence encoded by the Dna.
Think about It
- What are the reasons a nucleotide change in a gene for a protein might not have any event on the phenotype of that gene?
- Is it possible for an insertion of three nucleotides together after the fifth nucleotide in a poly peptide-coding gene to produce a poly peptide that is shorter than normal? How or how not?
A Benign Mutation
Since the first case of infection with homo immunodeficiency virus (HIV) was reported in 1981, about 40 1000000 people take died from HIV infection,[one] the virus that causes caused allowed deficiency syndrome (AIDS). The virus targets helper T cells that play a primal function in bridging the innate and adaptive immune response, infecting and killing cells unremarkably involved in the body's response to infection. There is no cure for HIV infection, but many drugs have been developed to slow or block the progression of the virus. Although individuals effectually the world may be infected, the highest prevalence amidst people fifteen–49 years old is in sub-Saharan Africa, where nearly ane person in 20 is infected, accounting for greater than 70% of the infections worldwide[two] (Effigy ii). Unfortunately, this is also a part of the earth where prevention strategies and drugs to treat the infection are the most lacking.

Effigy 2. HIV is highly prevalent in sub-Saharan Africa, but its prevalence is quite low in some other parts of the world.
In recent years, scientific interest has been piqued by the discovery of a few individuals from northern Europe who are resistant to HIV infection. In 1998, American geneticist Stephen J. O'Brien at the National Institutes of Wellness (NIH) and colleagues published the results of their genetic analysis of more than 4,000 individuals. These indicated that many individuals of Eurasian descent (up to 14% in some ethnic groups) have a deletion mutation, called CCR5-delta 32, in the gene encoding CCR5. CCR5 is a coreceptor found on the surface of T cells that is necessary for many strains of the virus to enter the host cell. The mutation leads to the product of a receptor to which HIV cannot effectively bind and thus blocks viral entry. People homozygous for this mutation have greatly reduced susceptibility to HIV infection, and those who are heterozygous accept some protection from infection as well.
Information technology is not clear why people of northern European descent, specifically, carry this mutation, but its prevalence seems to exist highest in northern Europe and steadily decreases in populations as ane moves south. Enquiry indicates that the mutation has been nowadays since before HIV appeared and may take been selected for in European populations every bit a result of exposure to the plague or smallpox. This mutation may protect individuals from plague (caused by the bacterium Yersinia pestis) and smallpox (acquired by the variola virus) because this receptor may also be involved in these diseases. The age of this mutation is a matter of debate, but estimates suggest it appeared between 1875 years to 225 years ago, and may have been spread from Northern Europe through Viking invasions.
This exciting finding has led to new avenues in HIV enquiry, including looking for drugs to block CCR5 bounden to HIV in individuals who lack the mutation. Although DNA testing to determine which individuals carry the CCR5-delta 32 mutation is possible, there are documented cases of individuals homozygous for the mutation contracting HIV. For this reason, DNA testing for the mutation is not widely recommended past public health officials so as non to encourage risky beliefs in those who deport the mutation. Nevertheless, inhibiting the binding of HIV to CCR5 continues to be a valid strategy for the development of drug therapies for those infected with HIV.
Causes of Mutations
Mistakes in the process of Dna replication can cause spontaneous mutations to occur. The mistake charge per unit of Deoxyribonucleic acid polymerase is one incorrect base per billion base pairs replicated. Exposure to mutagens can cause induced mutations, which are various types of chemical agents or radiation (Tabular array 1). Exposure to a mutagen can increase the rate of mutation more than than 1000-fold. Mutagens are often likewise carcinogens, agents that cause cancer. Notwithstanding, whereas nearly all carcinogens are mutagenic, non all mutagens are necessarily carcinogens.
| Table 1. A Summary of Mutagenic Agents | |||
|---|---|---|---|
| Mutagenic Agents | Way of Activity | Effect on DNA | Resulting Type of Mutation |
| Nucleoside analogs | |||
| 2-aminopurine | Is inserted in place of A just base of operations pairs with C | Converts AT to GC base pair | Point |
| five-bromouracil | Is inserted in place of T only base of operations pairs with G | Converts AT to GC base of operations pair | Signal |
| Nucleotide-modifying agent | |||
| Nitrous oxide | Deaminates C to U | Converts GC to AT base pair | Point |
| Intercalating agents | |||
| Acridine orange, ethidium bromide, polycyclic aromatic hydrocarbons | Distorts double helix, creates unusual spacing betwixt nucleotides | Introduces modest deletions and insertions | Frameshift |
| Ionizing radiation | |||
| X-rays, γ-rays | Forms hydroxyl radicals | Causes single- and double-strand Dna breaks | Repair mechanisms may introduce mutations |
| X-rays, γ-rays | Modifies bases (e.g., deaminating C to U) | Converts GC to AT base pair | Point |
| Nonionizing radiation | |||
| Ultraviolet | Forms pyrimidine (ordinarily thymine) dimers | Causes DNA replication errors | Frameshift or bespeak |
Chemic Mutagens
Diverse types of chemical mutagens interact directly with Deoxyribonucleic acid either by acting as nucleoside analogs or by modifying nucleotide bases. Chemicals called nucleoside analogs are structurally similar to normal nucleotide bases and can be incorporated into DNA during replication (Figure 3). These base analogs induce mutations because they ofttimes accept different base-pairing rules than the bases they replace. Other chemical mutagens can modify normal DNA bases, resulting in dissimilar base-pairing rules. For example, nitrous acid deaminates cytosine, converting information technology to uracil. Uracil then pairs with adenine in a subsequent round of replication, resulting in the conversion of a GC base pair to an AT base of operations pair. Nitrous acid also deaminates adenine to hypoxanthine, which base of operations pairs with cytosine instead of thymine, resulting in the conversion of a TA base pair to a CG base of operations pair.

Effigy iii. Click for a larger image. (a) 2-aminopurine nucleoside (2AP) structurally is a nucleoside analog to adenine nucleoside, whereas 5-bromouracil (5BU) is a nucleoside analog to thymine nucleoside. 2AP base pairs with C, converting an AT base of operations pair to a GC base pair afterward several rounds of replication. 5BU pairs with G, converting an AT base pair to a GC base pair later on several rounds of replication. (b) Nitrous acrid is a different type of chemical mutagen that modifies already existing nucleoside bases like C to produce U, which base of operations pairs with A. This chemical modification, as shown here, results in converting a CG base pair to a TA base pair.
Chemic mutagens known as intercalating agents work differently. These molecules slide between the stacked nitrogenous bases of the DNA double helix, distorting the molecule and creating atypical spacing betwixt nucleotide base pairs (Figure 4). As a consequence, during DNA replication, DNA polymerase may either skip replicating several nucleotides (creating a deletion) or insert actress nucleotides (creating an insertion). Either upshot may lead to a frameshift mutation. Combustion products like polycyclic aromatic hydrocarbons are particularly dangerous intercalating agents that can lead to mutation-caused cancers. The intercalating agents ethidium bromide and acridine orangish are commonly used in the laboratory to stain DNA for visualization and are potential mutagens.

Effigy 4. Intercalating agents, such as acridine, introduce atypical spacing between base pairs, resulting in DNA polymerase introducing either a deletion or an insertion, leading to a potential frameshift mutation.
Radiation
Exposure to either ionizing or nonionizing radiation can each induce mutations in Deoxyribonucleic acid, although by different mechanisms. Strong ionizing radiation like X-rays and gamma rays can cause single- and double-stranded breaks in the Deoxyribonucleic acid backbone through the formation of hydroxyl radicals on radiations exposure (Figure v). Ionizing radiation can as well modify bases; for example, the deamination of cytosine to uracil, analogous to the activeness of nitrous acid.[3] Ionizing radiation exposure is used to kill microbes to sterilize medical devices and foods, because of its dramatic nonspecific effect in damaging Deoxyribonucleic acid, proteins, and other cellular components (come across Using Physical Methods to Command Microorganisms).
Nonionizing radiation, similar ultraviolet light, is non energetic enough to initiate these types of chemic changes. However, nonionizing radiation tin can induce dimer formation between two next pyrimidine bases, commonly 2 thymines, within a nucleotide strand. During thymine dimer germination, the two side by side thymines become covalently linked and, if left unrepaired, both DNA replication and transcription are stalled at this indicate. Dna polymerase may proceed and replicate the dimer incorrectly, potentially leading to frameshift or point mutations.

Figure five. (a) Ionizing radiation may lead to the formation of unmarried-stranded and double-stranded breaks in the sugar-phosphate courage of Dna, as well every bit to the modification of bases (not shown). (b) Nonionizing radiation like ultraviolet light can lead to the formation of thymine dimers, which can stall replication and transcription and innovate frameshift or bespeak mutations.
Recollect about Information technology
- How does a base analog innovate a mutation?
- How does an intercalating agent innovate a mutation?
- What type of mutagen causes thymine dimers?
Deoxyribonucleic acid Repair
The process of DNA replication is highly accurate, merely mistakes can occur spontaneously or be induced past mutagens. Uncorrected mistakes can lead to serious consequences for the phenotype. Cells accept adult several repair mechanisms to minimize the number of mutations that persist.
Proofreading
Nigh of the mistakes introduced during Dna replication are promptly corrected by almost Deoxyribonucleic acid polymerases through a function chosen proofreading. In proofreading, the DNA polymerase reads the newly added base of operations, ensuring that it is complementary to the corresponding base in the template strand before adding the next one. If an incorrect base of operations has been added, the enzyme makes a cutting to release the wrong nucleotide and a new base is added.
Mismatch Repair
Some errors introduced during replication are corrected shortly after the replication machinery has moved. This machinery is chosen mismatch repair. The enzymes involved in this mechanism recognize the incorrectly added nucleotide, excise information technology, and supersede it with the correct base of operations. I example is the methyl-directed mismatch repair in East. coli . The DNA is hemimethylated. This means that the parental strand is methylated while the newly synthesized daughter strand is non. Information technology takes several minutes earlier the new strand is methylated. Proteins MutS, MutL, and MutH bind to the hemimethylated site where the wrong nucleotide is establish. MutH cuts the nonmethylated strand (the new strand). An exonuclease removes a portion of the strand (including the wrong nucleotide). The gap formed is then filled in by DNA pol Iii and ligase.
Repair of Thymine Dimers
Because the production of thymine dimers is common (many organisms cannot avoid ultraviolet calorie-free), mechanisms have evolved to repair these lesions. In nucleotide excision repair (likewise called dark repair), enzymes remove the pyrimidine dimer and replace it with the correct nucleotides (Figure six). In E. coli, the Dna is scanned by an enzyme complex. If a distortion in the double helix is establish that was introduced by the pyrimidine dimer, the enzyme complex cuts the sugar-phosphate backbone several bases upstream and downstream of the dimer, and the segment of DNA between these two cuts is then enzymatically removed. Deoxyribonucleic acid politician I replaces the missing nucleotides with the correct ones and Dna ligase seals the gap in the carbohydrate-phosphate backbone.
The directly repair (also chosen low-cal repair) of thymine dimers occurs through the process of photoreactivation in the presence of visible low-cal. An enzyme chosen photolyase recognizes the distortion in the DNA helix acquired by the thymine dimer and binds to the dimer. Then, in the presence of visible light, the photolyase enzyme changes conformation and breaks apart the thymine dimer, allowing the thymines to again correctly base pair with the adenines on the complementary strand. Photoreactivation appears to exist nowadays in all organisms, with the exception of placental mammals, including humans. Photoreactivation is particularly important for organisms chronically exposed to ultraviolet radiation, like plants, photosynthetic bacteria, algae, and corals, to prevent the aggregating of mutations acquired by thymine dimer formation.

Figure vi. Click for a larger paradigm. Bacteria have 2 mechanisms for repairing thymine dimers. (a) In nucleotide excision repair, an enzyme complex recognizes the distortion in the DNA circuitous around the thymine dimer and cuts and removes the damaged DNA strand. The correct nucleotides are replaced by DNA pol I and the nucleotide strand is sealed by DNA ligase. (b) In photoreactivation, the enzyme photolyase binds to the thymine dimer and, in the presence of visible light, breaks apart the dimer, restoring the base pairing of the thymines with complementary adenines on the opposite Deoxyribonucleic acid strand.
Think well-nigh It
- During mismatch repair, how does the enzyme recognize which is the new and which is the old strand?
- How does an intercalating agent introduce a mutation?
- What type of mutation does photolyase repair?
Identifying Bacterial Mutants
One common technique used to identify bacterial mutants is called replica plating. This technique is used to detect nutritional mutants, called auxotrophs, which have a mutation in a gene encoding an enzyme in the biosynthesis pathway of a specific food, such as an amino acrid. Equally a upshot, whereas wild-type cells retain the ability to grow normally on a medium lacking the specific nutrient, auxotrophs are unable to grow on such a medium. During replica plating (Figure 7), a population of bacterial cells is mutagenized and so plated as individual cells on a complex nutritionally complete plate and immune to grow into colonies. Cells from these colonies are removed from this master plate, ofttimes using sterile velvet. This velvet, containing cells, is then pressed in the same orientation onto plates of various media. At least one plate should also be nutritionally complete to ensure that cells are existence properly transferred between the plates. The other plates lack specific nutrients, allowing the researcher to discover various auxotrophic mutants unable to produce specific nutrients. Cells from the respective colony on the nutritionally complete plate can be used to recover the mutant for farther study.

Figure 7. Identification of auxotrophic mutants, like histidine auxotrophs, is done using replica plating. After mutagenesis, colonies that grow on nutritionally complete medium just non on medium lacking histidine are identified as histidine auxotrophs.
Think about Information technology
- Why are cells plated on a nutritionally complete plate in addition to nutrient-deficient plates when looking for a mutant?
The Ames Test
The Ames examination, developed past Bruce Ames (1928–) in the 1970s, is a method that uses leaner for rapid, inexpensive screening of the carcinogenic potential of new chemical compounds. The test measures the mutation rate associated with exposure to the compound, which, if elevated, may bespeak that exposure to this compound is associated with greater cancer risk. The Ames exam uses as the test organism a strain of Salmonella typhimurium that is a histidine auxotroph, unable to synthesize its own histidine because of a mutation in an essential gene required for its synthesis. After exposure to a potential mutagen, these bacteria are plated onto a medium defective histidine, and the number of mutants regaining the power to synthesize histidine is recorded and compared with the number of such mutants that arise in the absence of the potential mutagen (Effigy eight). Chemicals that are more mutagenic will bring about more than mutants with restored histidine synthesis in the Ames test. Because many chemicals are non directly mutagenic only are metabolized to mutagenic forms past liver enzymes, rat liver extract is commonly included at the commencement of this experiment to mimic liver metabolism. Later the Ames exam is conducted, compounds identified every bit mutagenic are further tested for their potential carcinogenic properties by using other models, including animal models like mice and rats.
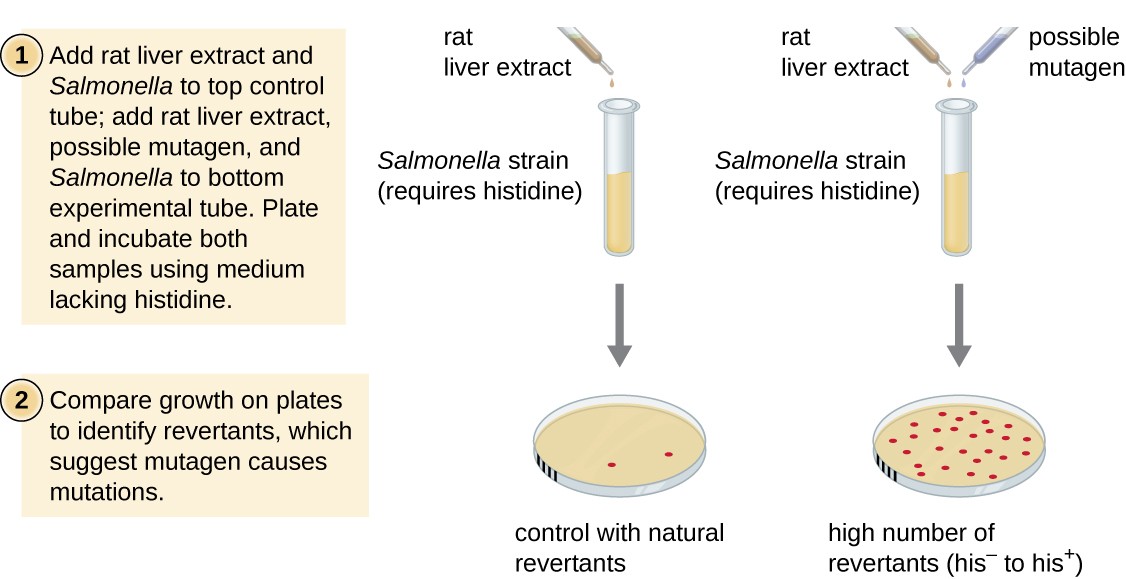
Figure 8. The Ames test is used to identify mutagenic, potentially carcinogenic chemicals. A Salmonella histidine auxotroph is used as the exam strain, exposed to a potential mutagen/carcinogen. The number of reversion mutants capable of growing in the absence of supplied histidine is counted and compared with the number of natural reversion mutants that ascend in the absenteeism of the potential mutagen.
Think about It
- What mutation is used as an indicator of mutation charge per unit in the Ames exam?
- Why can the Ames test piece of work every bit a test for carcinogenicity?
Cardinal Concepts and Summary
- A mutation is a heritable change in Dna. A mutation may lead to a change in the amino-acid sequence of a protein, peradventure affecting its function.
- A point mutation affects a unmarried base pair. A point mutation may cause a silent mutation if the mRNA codon codes for the same amino acrid, a missense mutation if the mRNA codon codes for a dissimilar amino acid, or a nonsense mutation if the mRNA codon becomes a cease codon.
- Missense mutations may retain office, depending on the chemistry of the new amino acid and its location in the protein. Nonsense mutations produce truncated and oft nonfunctional proteins.
- A frameshift mutation results from an insertion or deletion of a number of nucleotides that is non a multiple of iii. The change in reading frame alters every amino acid after the point of the mutation and results in a nonfunctional protein.
- Spontaneous mutations occur through Dna replication errors, whereas induced mutations occur through exposure to a mutagen.
- Mutagenic agents are frequently carcinogenic but not always. Nevertheless, nearly all carcinogens are mutagenic.
- Chemical mutagens include base analogs and chemicals that modify existing bases. In both cases, mutations are introduced subsequently several rounds of DNA replication.
- Ionizing radiation, such equally 10-rays and γ-rays, leads to breakage of the phosphodiester backbone of Deoxyribonucleic acid and can besides chemically alter bases to alter their base-pairing rules.
- Nonionizing radiation like ultraviolet light may introduce pyrimidine (thymine) dimers, which, during Deoxyribonucleic acid replication and transcription, may innovate frameshift or point mutations.
- Cells have mechanisms to repair naturally occurring mutations. DNA polymerase has proofreading activity. Mismatch repair is a process to repair incorrectly incorporated bases after Deoxyribonucleic acid replication has been completed.
- Pyrimidine dimers can also be repaired. In nucleotide excision repair (night repair), enzymes recognize the distortion introduced by the pyrimidine dimer and supersede the damaged strand with the right bases, using the undamaged Deoxyribonucleic acid strand as a template. Leaner and other organisms may likewise utilize direct repair, in which the photolyase enzyme, in the presence of visible calorie-free, breaks apart the pyrimidines.
- Through comparison of growth on the complete plate and lack of growth on media lacking specific nutrients, specific loss-of-office mutants called auxotrophs can be identified.
- The Ames test is an inexpensive method that uses auxotrophic bacteria to measure mutagenicity of a chemical compound. Mutagenicity is an indicator of carcinogenic potential.
Multiple Choice
Which of the following is a modify in the sequence that leads to formation of a terminate codon?
- missense mutation
- nonsense mutation
- silent mutation
- deletion mutation
Show Reply
Answer b. A nonsense mutation is a change in the sequence that leads to germination of a stop codon.
The formation of pyrimidine dimers results from which of the following?
- spontaneous errors past Dna polymerase
- exposure to gamma radiations
- exposure to ultraviolet radiations
- exposure to intercalating agents
Show Answer
Respond c. The formation of pyrimidine dimers results from exposure to ultraviolet radiations.
Which of the following is an instance of a frameshift mutation?
- a deletion of a codon
- missense mutation
- silent mutation
- deletion of 1 nucleotide
Show Answer
Reply a. The deletion of one nucleotide is an example of a frameshift mutation.
Which of the following is the type of DNA repair in which thymine dimers are direct broken down by the enzyme photolyase?
- straight repair
- nucleotide excision repair
- mismatch repair
- proofreading
Show Respond
Respond a. In a direct repair, thymine dimers are straight broken down by the enzyme photolyase.
Which of the post-obit regarding the Ames test is true?
- Information technology is used to identify newly formed auxotrophic mutants.
- It is used to place mutants with restored biosynthetic action.
- It is used to identify spontaneous mutants.
- It is used to identify mutants lacking photoreactivation activity.
Show Answer
Answer b. It is used to place mutants with restored biosynthetic activity.
Fill in the Blank
A chemical mutagen that is structurally similar to a nucleotide but has different base-pairing rules is called a ________.
Show Reply
A chemical mutagen that is structurally similar to a nucleotide but has unlike base-pairing rules is called a nucleoside analog.
The enzyme used in lite repair to split thymine dimers is called ________.
Testify Reply
The enzyme used in low-cal repair to split thymine dimers is chosen photolyase.
The phenotype of an organism that is most usually observed in nature is called the ________.
Show Respond
The phenotype of an organism that is most normally observed in nature is called the wild type.
Truthful/False
Carcinogens are typically mutagenic.
Recall nearly It
Why is it more than likely that insertions or deletions will be more detrimental to a cell than betoken mutations?
Why exercise you think the Ames exam is preferable to the use of animal models to screen chemical compounds for mutagenicity?
Critical Thinking
Below are several DNA sequences that are mutated compared with the wild-blazon sequence: iii′-T A C T M A C T Thou A C One thousand A T C-five′. Envision that each is a department of a DNA molecule that has separated in training for transcription, so you are only seeing the template strand. Construct the complementary Dna sequences (indicating 5′ and three′ ends) for each mutated DNA sequence, then transcribe (indicating five′ and 3′ ends) the template strands, and translate the mRNA molecules using the genetic code, recording the resulting amino acid sequence (indicating the N and C termini). What type of mutation is each?
| Mutated DNA Template Strand #1: iii′-T A C T One thousand T C T G A C G A T C-5′ | |
|---|---|
| Complementary Dna sequence: | |
| mRNA sequence transcribed from template: | |
| Amino acid sequence of peptide: | |
| Type of mutation: | |
| Mutated Dna Template Strand #2: 3′-T A C G G A C T G A C K A T C-five′ | |
|---|---|
| Complementary DNA sequence: | |
| mRNA sequence transcribed from template: | |
| Amino acid sequence of peptide: | |
| Blazon of mutation: | |
| Mutated DNA Template Strand #iii: 3′-T A C T G A C T G A C T A T C-5′ | |
|---|---|
| Complementary DNA sequence: | |
| mRNA sequence transcribed from template: | |
| Amino acid sequence of peptide: | |
| Type of mutation: | |
| Mutated DNA Template Strand #iv: iii′-T A C G A C T G A C T A T C-5′ | |
|---|---|
| Complementary DNA sequence: | |
| mRNA sequence transcribed from template: | |
| Amino acrid sequence of peptide: | |
| Type of mutation: | |
Source: https://courses.lumenlearning.com/microbiology/chapter/mutations/
Posted by: acostaficky2002.blogspot.com


0 Response to "How Are Mutations Repaired Bio"
Post a Comment